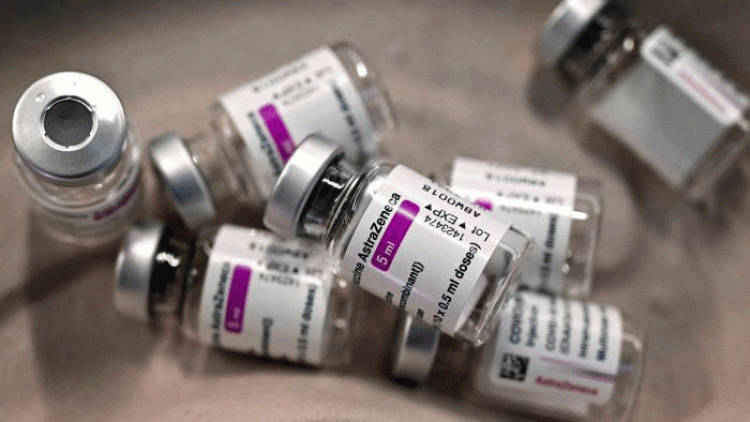
A third dose of AstraZeneca's Covid vaccine, named Covishield in India, increases antibody response to the new vaccine-evading Omicron variant, according to a new trial published by the British-Swedish drugmaker on Thursday.
The trial showed the third dose booster also increases the immune response to Beta, Delta, Alpha, and Gamma SARS-CoV-2 variants. The results of both were observed among individuals previously vaccinated with either AstraZeneca, Vaxzevria in the UK, or an mRNA vaccine.
"These important studies show that a third dose of Vaxzevria after two initial doses of the same vaccine, or after mRNA or inactivated vaccines, strongly boosts immunity against Covid-19," said Professor Sir Andrew J. Pollard, chief investigator and director of the Oxford Vaccine Group at the University of Oxford, in a statement.
Also read| No need for 4th Covid jab yet: UK experts
"The Oxford-AstraZeneca vaccine is suitable as an option to enhance immunity in the population for countries considering booster programmes, adding to the protection already demonstrated with the first two doses," he added. A recent study showed that AstraZeneca's shot increased antibodies when given as a booster after initial vaccination with its own shot or mRNA-based Pfizer's. But the study also noted that mRNA vaccines by Pfizer and Moderna gave the biggest boost to antibodies when given as a booster dose.
AstraZeneca said that the new data adds to the growing body of evidence supporting Vaxzevria as a third dose booster irrespective of the primary vaccination schedules tested, adding that it is submitting the additional data to health authorities.
"Given the ongoing urgency of the pandemic and Vaxzevria's increased immune response to the Omicron variant, we will continue to progress regulatory submissions around the world for its use as a third dose booster," said Mene Pangalos, Executive Vice President, BioPharmaceuticals R&D, AstraZeneca.
Meanwhile, AstraZeneca on Thursday also welcomed the US government's announcement for the purchase of an additional 500,000 doses of its antibody drug Evusheld. The US Food and Drug Administration (FDA) had, last month, granted emergency use authorisation to Evusheld -- an injectable monoclonal antibody cocktail of tixagevimab co-packaged with cilgavimab - for Covid-19 among people with weakened immune systems.
Also read| Covid-recovered at 3-5 times more Omicron reinfection risk: WHO
It is the first antibody treatment against Covid-19 that has won an EUA from the drug regulator. Evusheld is also effective against the Omicron variant. Delivery of the additional 500,000 doses is anticipated in the first quarter of 2022, the company said in a statement.
This follows the previous government agreement for the purchase of 700,000 doses of Evusheld.