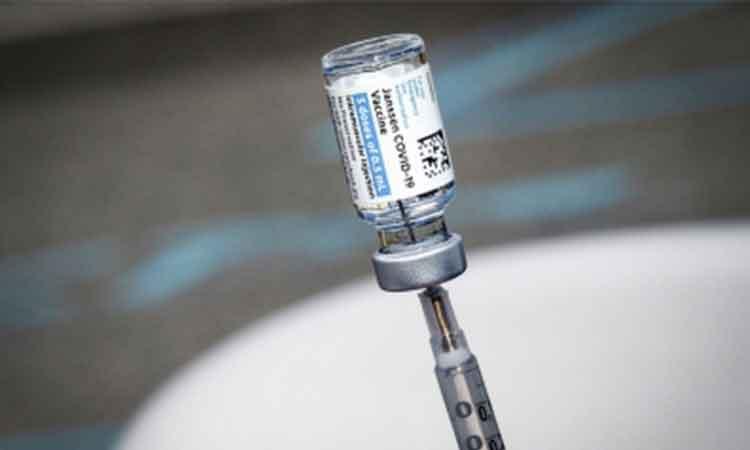
The US Food and Drug Administration (FDA) announced that it has limited the authorised use of the Johnson & Johnson Covid-19 vaccine, Janssen, to individuals 18 years of age and older who cannot or will not get other jabs, over the risk of rare blood clots.
"After conducting an updated analysis, evaluation and investigation of reported cases, the FDA has determined that the risk of thrombosis with thrombocytopenia syndrome (TTS), a syndrome of rare and potentially life-threatening blood clots in combination with low levels of blood platelets with onset of symptoms approximately one to two weeks following administration of the Janssen Covid-19 vaccine, warrants limiting the authorisd use of the vaccine," the FDA said in a statement on Thursday.
"Our action reflects our updated analysis of the risk of TTS following administration of this vaccine and limits the use of the vaccine to certain individuals," Xinhua news agency quoted Peter Marks, Director of the FDA's Center for Biologics Evaluation and Research, as saying.
He said the FDA has been closely monitoring the Janssen vaccine and occurrence of TTS following its administration and has used updated information from its safety surveillance systems to revise the authorization.
Also read | India reports marginal rise in Covid cases
The Johnson & Johnson Covid-19 vaccine was authorized for emergency use in the US on February 27, 2021.
Through March 18 this year, the FDA and the Centers for Disease Control and Prevention have identified 60 confirmed TTS cases, including nine fatal cases.