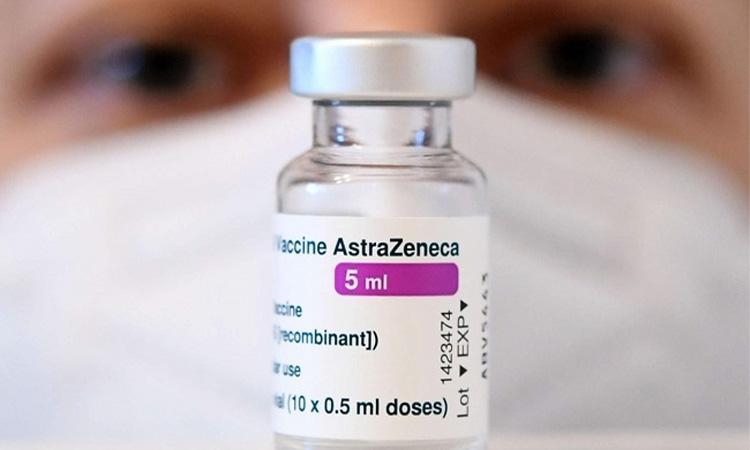
Faced with a vaccine evading Omicron variant, Oxford university and AstraZeneca announced that they have begun the work to develop a Covid shot that specifically targets the new strain.
Early this month, all major drug makers including Pfizer, Moderna, Johnson and Johnson, and AstraZeneca had announced plans to quickly investigate and adapt their shots to the highly mutated Omicron variant.
"Like with many previous variants of concern, and together with our partners AstraZeneca, we have taken preliminary steps in producing an updated vaccine in case it is needed," Sandy Douglas, a research group leader at Oxford, was quoted as saying to the Financial Times.
"Adenovirus-based vaccines [such as that made by Oxford/AstraZeneca] could in principle be used to respond to any new variant more rapidly than some may previously have realised. [They have] really important advantages, especially where need and logistical challenges are greatest," he added.
Also Read | No evidence to suggest existing vaccines don't work on Omicron: Govt
A study published in the journal The Lancet on Monday showed that the protection offered by the Oxford-AstraZeneca Covid-19 vaccine, named Covishield in India, declines after three months of receiving two doses.
The findings by a team of researchers led by University of Edinburgh suggest that booster programmes are needed to help maintain protection from severe disease.
The results are consistent with several studies rendering two doses of nearly all widely used vaccines less effective against the highly transmissible variant. However, a third messenger RNA shot has shown to increase antibody levels.
"Together with Oxford university, we have taken preliminary steps in producing an Omicron variant vaccine, in case it is needed and will be informed by emerging data," AstraZeneca was quoted as saying by the newspaper.
Also Read | Bharat Biotech seeks approval for Phase 3 trials of intranasal vaccine
The AstraZeneca shot, with partnership from Oxford and India's Serum Institute, was widely deployed with more than 2 billion doses globally - the majority being supplied in poorer nations.
However, several countries restricted its use after the emergence of a rare side effect involving blood clots, the report said.
In a yet to be peer-reviewed paper posted on preprint on Tuesday, Douglas's team highlighted the speed at which it would be possible to make a new adenovirus-vectored vaccine such as AstraZeneca's at scale.
According to researchers, "their work will help the vaccines to hit the 100-day development target, taking little more than three months from pathogen identification to mass production, potentially including the distribution of millions of doses from manufacturing sites globally", the report said.
Meanwhile, US top infectious disease expert Dr Anthony Fauci, this week, said that existing vaccines and booster shots against Covid are sufficient to prevent Omicron infections. He advised against changing the vaccines to fight the new, highly contagious strain of the virus at this time.
"Our booster vaccine regimens work against Omicron. At this point, there is no need for a variant-specific booster," Fauci said.
Data from the UK Health Security Agency showed that a booster dose increases protection against symptomatic disease to 75 per cent.
Even the European Medicines Agency has warned that it would take time to reach a global scientific consensus on whether Omicron-targeted shots are needed, the report said.