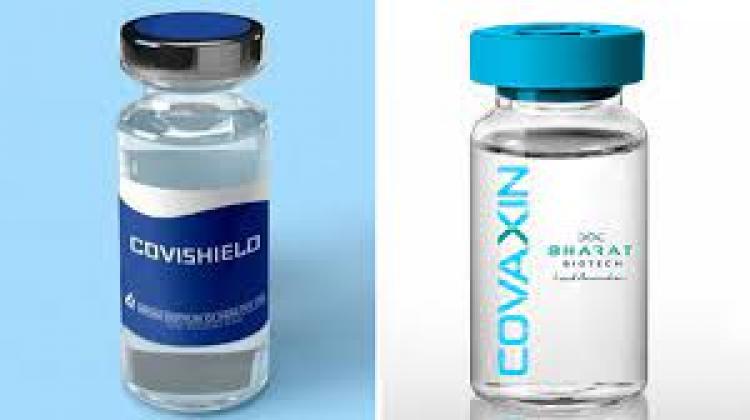
The Drugs Controller General of India (DCGI) has given the approval to conduct a study on mixing of Covishield and Covaxin vaccines. The step has been taken to boost the mass vaccination drive against the coronavirus across the nation.
The DCGI has said that the study and its clinical trials will be conducted by Christian Medical College in Vellore.
The study aims to find out whether a person can be given two different vaccine shots -- one each of Covishield and Covaxin -- to be fully vaccinated against the coronavirus disease, instead of the existing practice of administering twin shots of the same vaccine.
Also Read | Wanted something concrete: SC to CBI on weekly report into Dhanbad judge's murder
A Subject Expert Committee of the Central Drugs Standard Control Organisation (CDSCO) on July 29, recommended for conducting this study on mixing of the two shots.
The expert committee recommended granting permission to CMC, Vellore, for conducting the Phase-4 clinical trial which may conduct the study on 300 healthy volunteers by administered a dose of each of Covaxin and Covishield.
Also Read | Oxford-AstraZeneca Covid jab linked to low platelet count: Study
However, the DCGI's approval for the study on mixing the vaccines is different from the recent study of the Indian Council of Medical Research (ICMR) which concluded that combining two different shots is "safe and effective".
The ICMR had analysed an "accidental" mixing of Covishield and Covaxin.